Research
Our research is centered in the unique chemical and physical phenomena that occur at surfaces and interfaces of solid materials.
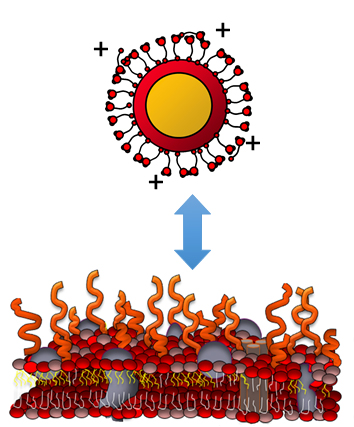 Sustainable Nanotechnology: Our efforts in this area are primarily associated with the Center for Sustainable Nanotechnology (CSN), a Center for Chemical Innovation funded by the National Science Foundation. In the Center for Sustainable Nanotechnology we aim to understand, predict, and control the specific chemical and physical interactions between nanoparticles and and living systems via a molecular level, chemistry-centered approach. CSN research in the Hamers group is specifically targeted toward (1) using the power of chemistry to design and synthesize functional, environmentally benign nanoparticles for emerging and future nano-enabled technologies, (2) development and implementation of state-of-the-art techniques for characterizing nanomaterials in aqueous and/or biological systems.
Sustainable Nanotechnology: Our efforts in this area are primarily associated with the Center for Sustainable Nanotechnology (CSN), a Center for Chemical Innovation funded by the National Science Foundation. In the Center for Sustainable Nanotechnology we aim to understand, predict, and control the specific chemical and physical interactions between nanoparticles and and living systems via a molecular level, chemistry-centered approach. CSN research in the Hamers group is specifically targeted toward (1) using the power of chemistry to design and synthesize functional, environmentally benign nanoparticles for emerging and future nano-enabled technologies, (2) development and implementation of state-of-the-art techniques for characterizing nanomaterials in aqueous and/or biological systems.
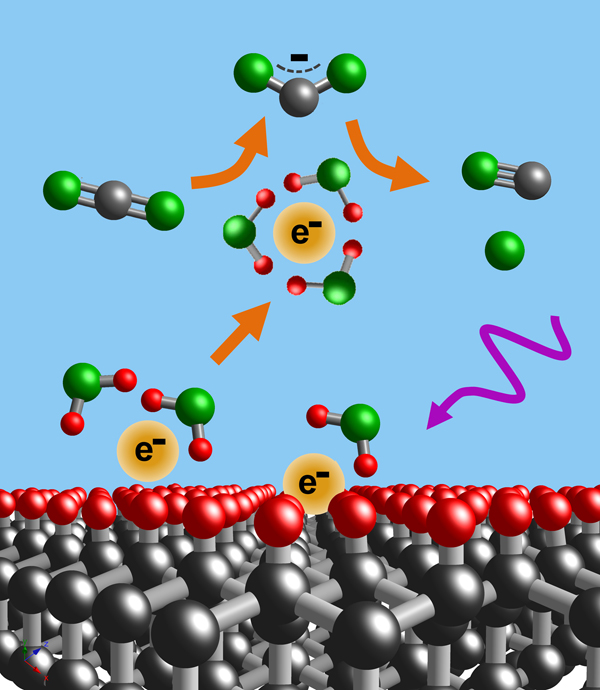 Extreme Electrochemistry: Electrochemistry and photoelectrochemistry are commonly limited by the stability range of water. We are conducting reseach aimed at enabling new electrochemical processes outside the water stability limits of water. Particular targets of interest include
Extreme Electrochemistry: Electrochemistry and photoelectrochemistry are commonly limited by the stability range of water. We are conducting reseach aimed at enabling new electrochemical processes outside the water stability limits of water. Particular targets of interest include
- Reduction of CO2 to CO
- Conversion of N2 to NH3
- Formation of hydroxyl radicals for water purification.
Much of this work exploits the unique electrical and chemical properties of diamond as an ultra-stable electrode materials. Contrary to common perception, diamond in thin-film and/or nanoparticle form is an inexpensive, infinitely-reusable electrode material with many unique chemical and physical properties.
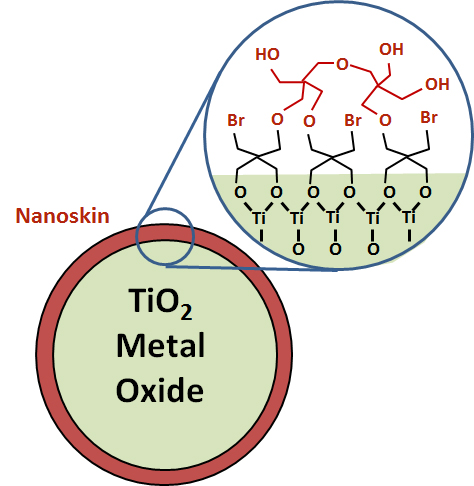 Nanoskins: Oxide materials form the basis of a wide range of technologies, ranging form photovoltaic and photocatalytic energy conversion to cathodes for energy storage. One of the key challenges in this field is making interfaces between inorganic materials (especially metal oxides) and organic materials that are stable in aqueous media. We have a range of projects aimed at manipulating the surface chemistry of materials to enhance their chemical stability and/or photophysical properties by forming laterally cross-linked “nanoskins” on the outside of the nanoparticle. These nanometer-thin skins can then serve as handles for linking other active groups such as catalysts or light-harvesting molecules to the oxides. Ultimately the goal is to make highly stable, functional organic-inorganic interfaces.
Nanoskins: Oxide materials form the basis of a wide range of technologies, ranging form photovoltaic and photocatalytic energy conversion to cathodes for energy storage. One of the key challenges in this field is making interfaces between inorganic materials (especially metal oxides) and organic materials that are stable in aqueous media. We have a range of projects aimed at manipulating the surface chemistry of materials to enhance their chemical stability and/or photophysical properties by forming laterally cross-linked “nanoskins” on the outside of the nanoparticle. These nanometer-thin skins can then serve as handles for linking other active groups such as catalysts or light-harvesting molecules to the oxides. Ultimately the goal is to make highly stable, functional organic-inorganic interfaces.
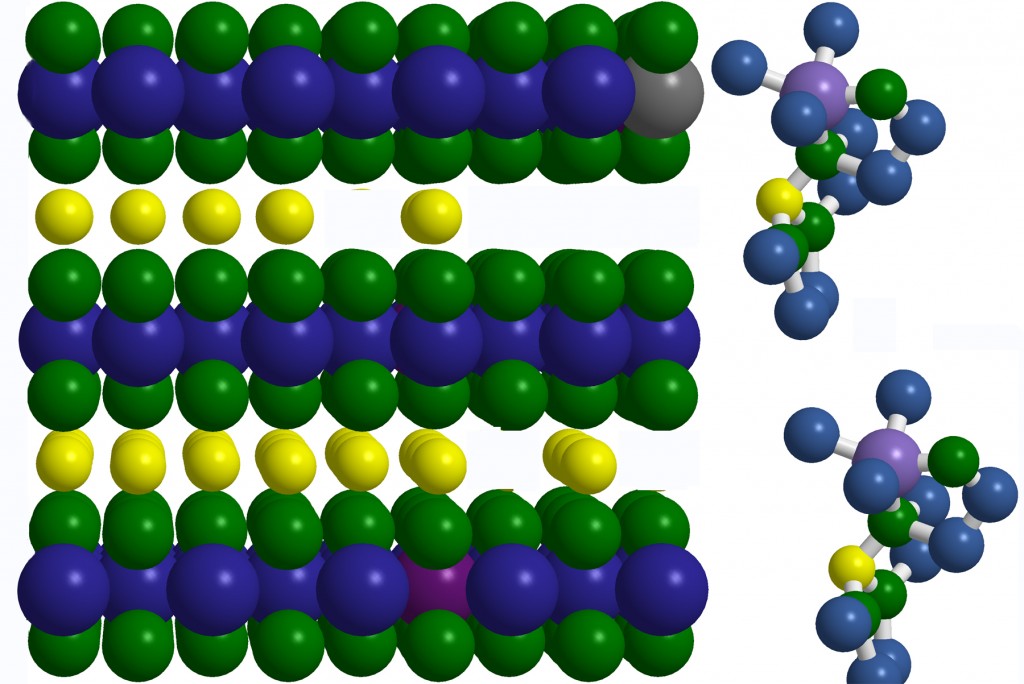 Energy Storage Materials: The explosive growth in mobile electronics and in energy-efficient hybrid/electric vehicles is largely enable through advances in the ability to store energy. Lithium-ion batteries are of particular interest. Ongoing studies are investigating chemical stabilization of cathode materials and investigating next-generation electrolytes. In collaboration with Dow Chemical, we are investigating how to use ultra-thin organic coatings to improve the performance of lithium-ion battery cathode materials. Next-generation cathode materials also require next-generation electrolytes able to withstand harsh electrochemical conditons. In collaboration with Silatronix, we are researching the chemical stability of new electrolytes and identifying the fundamental mechanistic pathways that lead to electrolyte breakdown, with the goal of making even more stable electrolytes for the future. Silatronix is a ~15-person startup company co-founded in 2008 by Prof. Hamers and Prof. Robert West. Hamers serves as the Chief Technical Officer and has a financial interest in the outcome of the work with Silatronix. To minimize conflicts of interest and protect the rights of students, UW research with Silatronix is managed in accordance with a University of Wisconsin-approved Management Plan.
Energy Storage Materials: The explosive growth in mobile electronics and in energy-efficient hybrid/electric vehicles is largely enable through advances in the ability to store energy. Lithium-ion batteries are of particular interest. Ongoing studies are investigating chemical stabilization of cathode materials and investigating next-generation electrolytes. In collaboration with Dow Chemical, we are investigating how to use ultra-thin organic coatings to improve the performance of lithium-ion battery cathode materials. Next-generation cathode materials also require next-generation electrolytes able to withstand harsh electrochemical conditons. In collaboration with Silatronix, we are researching the chemical stability of new electrolytes and identifying the fundamental mechanistic pathways that lead to electrolyte breakdown, with the goal of making even more stable electrolytes for the future. Silatronix is a ~15-person startup company co-founded in 2008 by Prof. Hamers and Prof. Robert West. Hamers serves as the Chief Technical Officer and has a financial interest in the outcome of the work with Silatronix. To minimize conflicts of interest and protect the rights of students, UW research with Silatronix is managed in accordance with a University of Wisconsin-approved Management Plan.